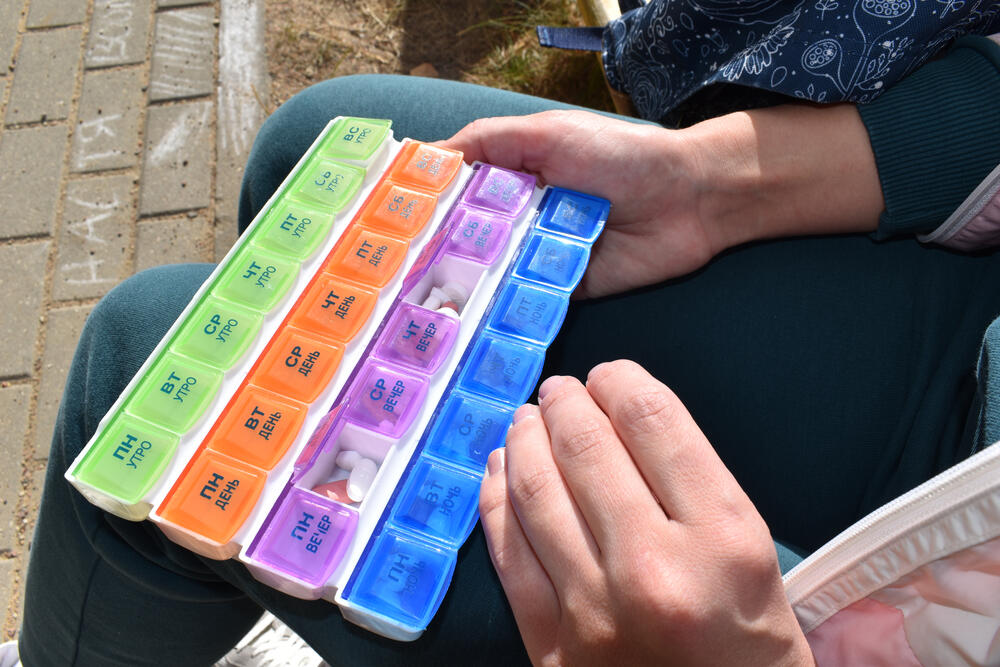
Multidrug-resistant Tuberculosis can be treated at home - Doctors Without Borders supports new pilot programme for the treatment of tuberculosis in Poland
13 December 2022
Warsaw, 13 December 2022 – Médecins Sans Frontières/Doctors Without Borders (MSF) announces support for a pilot multidrug-resistant tuberculosis (MDR-TB) treatment programme, aimed at providing a shorter, safer, more effective treatment regimen that patients can follow from their own homes. The programme has the potential to increase the standard of MDR-TB treatment and to significantly improve patients' quality of life.
The main goal of the programme coordinated by the Institute of Tuberculosis and Lung Diseases in Warsaw (IGiChP), and supported by, among others, the World Health Organization (WHO) and MSF, is to introduce a model for treating multidrug-resistant tuberculosis in an outpatient setting, using new drug therapies. This outpatient approach is the WHO's guiding standard.
– The treatment of MDR-TB should focus on the patient – says Joanna Ladomirska, MSF medical coordinator in Poland. – In the vast majority of cases, it can and should be carried out at home, with the support of telemedicine solutions. Being in one's own home with the possibility of social and professional activity improves the physical and mental condition of patients and increases the effectiveness of treatment.
Currently, treatment of MDR TB in Poland can take as long as 24 months and takes place in hospitals. Shifting to an outpatient model of care will not only reduce the strain on the healthcare system but will allow patients to live full and uninterrupted family, social and professional lives. The use of new, effective and less toxic treatment schema in the pilot programme, will prevent the infectiousness of TB patients and the transmission of TB to other people.
In this new programme, MSF and WHO are recommending short (six or nine months), oral regimens containing Bedaquiline. The six-month regime is comprised of Bedaquiline, Pretomanid, Linezolid and Moxifloxacin. The nine-month regimen includes Bedaquiline, Fluoroquinolones (Levofloxacin or Moxifloxacin) and Linezolid combined with Clofazimine and Cycloserine.
This pilot programme also aims to ensure continuity of treatment for Ukrainian refugees who were diagnosed with tuberculosis while still in Ukraine. For many of them, already forced to leave their own country, the prospect of a long-term hospital admission can be extremely difficult, especially parents who need to care for young children at home. Since July, MSF have been reaching out to these people directing them to the adequate medical facilities where they can continue their treatment started in Ukraine. They also receive social and psychological support from MSF, which is an important part of the overall treatment.
The organisation supports Polish medical personnel with training on the effective implementation of this treatment, and in the initial period of the pilot programme provided all the necessary medicines for 35 patients. Support is also provided by the WHO; including the donation of medicines for a further 100 treatments, funding for diagnostic equipment and ICT solutions to support outpatient treatment.
– The treatment of infectious diseases is always a complex process – explains Adam Nowiński, MD, PhD, coordinator of the pilot project from the Institute of Tuberculosis and Lung Diseases. – Not only is it important to have access to modern, sometimes very expensive drugs and efficient diagnostics, but social aspects also are important. That's why we appreciate the support of MSF, an organisation which has experience in providing aid to refugees.
MSF will continue existing activities: patient outreach and referrals, as well as health education activities. The first workshop for Polish medical personnel organised by IGiChP and WHO, with the participation of MSF, was held on October 21.
– As a medical humanitarian organisation with a global reach, we have extensive experience in treating tuberculosis, which is unfortunately still prevalent in many parts of the world. Only last year MSF started to treat over 1,800 new patients for MDR-TB . In last decade it was over 10 000 patients. – says Joanna Ladomirska. – This project is an opportunity to change the approach to treating drug-resistant tuberculosis in Poland, which can fundamentally improve the quality of life of patients.